In June 2022, Persistence Market Research presented a market outlook of orally disintegrating tablet (ODT) for the next years (2022 - 2025).
According to this research “The ODT Market is likely to have compound annual growth rate (CAGR) of 8.5% over the forecast period. One of the primary factors driving the growth of Orally Disintegrating Tablets is that these tablets are increasingly being used as an alternative to traditional tablets or capsules”.[1]
ODTs are namely orally disintegrating tablets or orodispersible tablets containing a medicinal substance and disintegrating within a matter of seconds in the mouth without any intake of externally administered liquids. Thus, the active pharmaceutical ingredient (API) is delivered via the mouth mucosa avoiding the first-pass metabolism leading to an increased bioavailability for enzymatic sensitive APIs as example.[2]
ODTs are defined by the pharmacopoeias as weighing maximal 500 mg and disintegrating in less than 30 seconds (American pharmacopoeia /USP) or 180 seconds (European pharmacopoeia/EP) in 2 mL of saliva.
Due to their fast disintegration in the mouth, ODTs are particularly interesting for pediatric/geriatric patient populations. They are easy to administer and are less likely to be rejected by the patient. Another characteristic of ODTs is their mouthfeel. In most of the formulations, sugar based excipients are used providing sweetness and a cooling effect resulting in a kind of taste masking which are very well appreciated by pediatric or geriatric patients.
ODTs are as well a good alternative for patients suffering from dysphagia.[3,4]
In a publication from 2020, it was found that around 1 million individuals have swallowing issues.
In that purpose, ODTs as fast dissolving tablets are a valuable oral dosage form improving patient acceptance and adherence of these medicines.
Another non negligible aspect is that ODTs can be taken without any liquid. It is an ideal solid dosage form for people living in regions in which drinkable water is rare or for people travelling frequently and not always having water on hand.
Major technologies used for the production of ODTs are freeze-drying/lyophilization and direct compression.
During lyophilization, a solution or a suspension containing API and excipients is prepared and cooled below its triple point (phase in which solid, liquid and gas phases can coexist). [5,6]
This leads to the sublimation of liquid. After this, the powder obtained is dried in two drying steps (sublimation of ice and removal of absorbed water).
The lyophilization can be done directly in a mold or blisters.
Typical excipients used in those formulations are polyols, polymers, suspension agents, preservatives, buffers and water as the solvent.
One of the advantages that lyophylization results in a porous structure and thus in a very fast disintegration. However, the product obtained will be very sensitive to breaking forces as it will be very brittle in nature.
A more easy and faster way to prepare ODTs is by direct compression.
In contrast to lyophilization, no specific equipment is required besides a tableting press.
The powders can be mixed together and directly compressed into tablets.
The obtained tablets are more stable than lyophilized ones.
Typical excipients used are disintegrants and sugar based water soluble excipients especially polyols providing a sweet taste and pleasant mouthfeel. Lubricant and glidant can be added to improve the compression step.
Shin-Etsu’s low-substituted hydroxypropyl cellulose (L-HPC) range as a multi-functional excipient showing good balance between binding and disintegrating properties is a promising excipient for fast disintegrating tablets. The NBD-022 grade in particular is very suitable for the formulation of ODTs.
NBD-022 is very compressible and has a very fast liquid absorption over the other L-HPC grades. It is also known for disintegrating the tablet into small particles leading to a fast dissolution as well as a fast disintegration. The tablets obtained using L-HPC grades have good tablet hardness and thus are stable in packaging.
Additionally, L-HPC can be combined with polyols and fulfill the requirements of the pharmacopoeias for the disintegration. Another approach to minimize the number of excipients used in the formulation is the usage of co-processed excipients.
The American Pharmaceutical Review summarizes co-processed excipients as: “a combination of two or more excipients obtained by physical co-processing that does not lead to the formation of covalent bonds. Co-processed excipients have functionalities that cannot be achieved through sample blending.” [7]
Shin-Etsu developed a multi-compendial co-processed excipient based on L-HPC as disintegrant, mannitol as filler and polyvinylalcohol (PVA) as binder under the name SmartEx® Plus. Due to this unique composition, SmartEx® Plus is a free flowing excipient showing high compressibility and stability ideal for ODTs produced by direct compression (DC).
Mannitol being the preferred choice of filler, adds a sweet taste and cooling effect.
L-HPC on the other hand is providing a fast disintegration into small particles and thus leading to a smooth mouthfeel. PVA is a binder used during the processing to provide good flowability without impacting the disintegration time. Using a co-processed excipient simplifies the formulation because in most cases, besides the API and the co-processed material, only lubricant is needed for a DC process.

Figure 1. Disintegration of SmartEx® Plus tablet.
From a processing point of view, one major challenge in ODT formulations made by direct compression is to maintain a good balance between the tablet hardness and the disintegration time.
Indeed, the tablet hardness or tensile strength should be high enough to avoid any damage of the tablets during further processing like blistering for example. One the other hand, a disintegration time of less than 180 sec (disintegration limit given by the European Pharmacopeia or EP for ODT) or 30 sec (according to the US Pharmacopeia or USP) is often times a difficult challenge to overcome. “Too hard tablets” may not match those requirements.
A formulation trial based on acetaminophen (analgesic) and SmartEx® Plus was performed and a very good balance between the disintegration time and tensile strength of the tablet could be obtained.
The formulation and results are shown in table 1 and figure 2. The disintegration was performed according to the USP method in water and a disintegration time below 30 seconds was observed.
| Material | w/w (%) |
| Acetaminophen | 0.0 to 50.0 % |
| SmartEx® Plus | 99.5 to 49.5 % |
| Magnesium stearate | 0.5 % |
Table 1. Formulation of acetaminophen ODT.
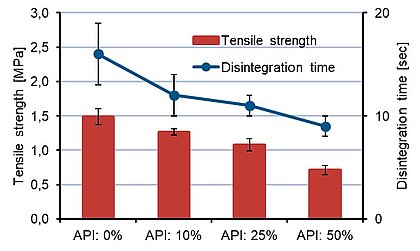
Figure 2. Results of tensile strength versus disintegration time.
ODMTs gained an increased interest in the last years as they offers a very flexible and easy to develop approach when looking into heterogeneous patient groups from children to senior citizens. Oral disintegrating mini-tablets are a combination of several advantages coming from the mini-tablet dosage form in terms of stability, accurate dosing and the advantage of the fast disintegration of ODTs. Mini-tablets are tablets smaller than 3 mm.
One of the challenges here is how to analyze the disintegration time of these dosage forms. Standard disintegration testers will lead to a very high standard deviation of the results and capture of the real disintegration time when the tablets are falling between the fine mesh of the tester.
One solution provided by the University of Düsseldorf in 2012 was the use of the texture analyzer.[8,9]
The tablet is attached to the probe with double-side tape (figure 3). In the vessel underneath the probe a wetted filter paper is placed. The probe is moved down until a pre-defined force on the probe is reached. The oral disintegrating mini-tablet gets into contact with the water, and as moisture softens the tablet, it cannot withstand the constant force applied. The probe will move further down and the tablet will disintegrate. The time of onset and offset of movement after contact with water can be used to extrapolate the disintegration time. With this method, more accurate results were achieved to determine the disintegration time of the ODMT.
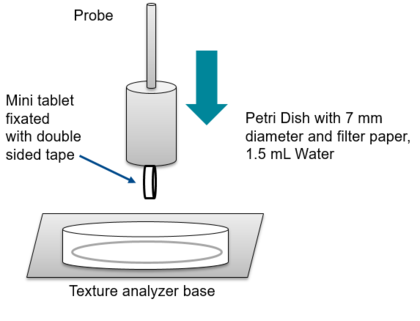
Figure 3. Set up texture analyzer for the analysis of disintegration of ODMT.
In summary, ODTs and ODMTs can be considered as dosage forms that improve patient adherence to medicines. Indeed, for those dosage forms, a special focus is given to palatability (smooth feeling in the mouth) and reducing the tablet size to mini-tablets provides the flexibility to adjust the dosage of the active ingredient to the target population (different pediatric segments or age population). Currently, several ODT products are available on the market. Some references and applications are listed in table below.
| Active ingredient | Brand name | Application |
| Loratidine | Claritin | Antihistaminic |
| Mirtapazin | Remeron | Antidepressant |
| Olanzatpine | Zyprexa | Antipsychotic |
| Zolmitriptan | Zomig | Antimigraine |
Table 2. Examples of currently marketed ODTs.
[1] Persistence market Research Website - https://www.persistencemarketresearch.com/market-research/orally-disintegrating-tablet-market.asp (accessed June 2023)
[2] Chinwala M. Recent Formulation Advances and Therapeutic Usefulness of Orally Disintegrating Tablets (ODTs). Pharmacy (Basel). 2020;8(4):186 - https://pubmed.ncbi.nlm.nih.gov/33050437/
[3] Schiele JT, Quinzler R, Klimm HD, Pruszydlo MG, Haefeli WE. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4):937-948 - https://pubmed.ncbi.nlm.nih.gov/23052416/
[4] Skarbinski, Kristina F. MSN, FNP-BC; Glennon, Elizabeth MSN, FNP-BC. Dysphagia: A review. The Nurse Practitioner 2020; 45(7):p 9-16 - https://journals.lww.com/tnpj/fulltext/2020/07000/dysphagia__a_review.2.aspx
[5] Brecht Vanbillemont, Thomas De Beer, Model-based optimization of the primary drying phase of oral lyophilizates, International Journal of Pharmaceutics: X 2020, Volume 2 - https://www.sciencedirect.com/science/article/pii/S2590156720300190
[6] Tascón-Otero E, Torre-Iglesias P, García-Rodríguez J.J, Peña M, Álvarez-Álvarez C. Enhancement of the Dissolution Rate of Indomethacin by Solid Dispersions in Low-substituted Hydroxypropyl Cellulose. Indian J Pharm Sci 2019;81(5):824-833 - https://www.ijpsonline.com/articles/enhancement-of-the-dissolution-rate-of-indomethacin-by-solid-dispersions-in-lowsubstituted-hydroxypropyl-cellulose-3696.html
[7] American Pharmaceutical Review Website, https://www.americanpharmaceuticalreview.com/pfu/7964385/soids/1402524/Excipient_Search/Co-processed (accessed June 2023)
[8] Stoltenberg, Entwicklung und Charakterisierung einer neuen festen Darreichungsform für die Pädiatrie, PhD Thesis, Universität Düsseldorf, 2012.
[9] Dor PJ, Fix JA. In vitro determination of disintegration time of quick-dissolve tablets using a new method. Pharm Dev Technol. 2000;5(4):575-577 - https://pubmed.ncbi.nlm.nih.gov/11109257/

Solid pharmaceutical dosage forms, such as tablets and capsules, are the most used drug delivery systems, favored for their ease of production and patient preference.
Read more
Oral solid dosage forms (OSD) present numerous options, over semi-solids and liquids, to enhance patient acceptance to medicines.
Read more
Nitrosamine Contamination of Drug Products is a topic of concern for pharmaceutical companies. As such, it deserves our utmost attention.
Read more